European Commission Gives Final Approval To Rollout Of The Pfizer BioNTech Vaccine
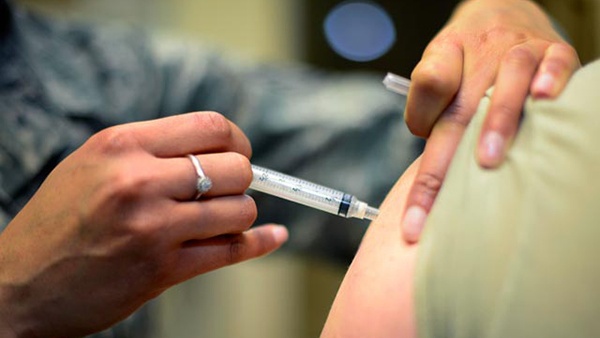
The authorisation makes the jab legally binding across all member states, and follows the conditional approval by the European Medicines Agency yesterday.
Its rollout here is expected in the 'coming days', with the first 10,000 doses due to arrive before the end of the year.
Dr Lorraine Nolan, chief executive of the Health Products Regulatory Authority, says the EMA's approval is reassuring:
"This thorough evaluation means that, Irish, and all EU citizens can be assured of the safety and effectiveness of this vaccine, and that it meets the necessary quality standards. However, from a regulatory perspective, we will continue to collect and analyse data on the safety and effectiveness of this vaccine as part of an extensive monitoring programme to ensure this, and any additionally authorised vaccines remain safe and effective."